JDRF UK
Revolutionising the treatment of type 1 diabetes
JDRF’s funded research and advocacy has resulted in a cutting-edge technological breakthrough for the treatment of type 1 diabetes (T1D), which is expected to transform the lives of millions of children and adults across the UK and globally over the next five years.
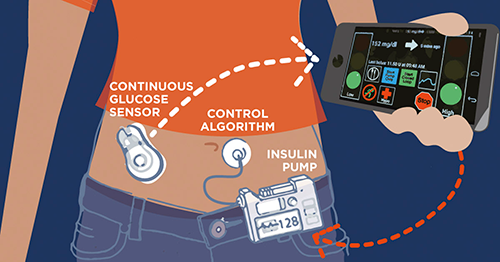 The “artificial pancreas” hybrid closed loop (HCL) system represents the most significant breakthrough in T1D treatment since the discovery of insulin a century ago. The technology combines the use of an app, a continuous glucose monitor and an insulin pump to deploy an algorithm that learns each individual’s insulin needs and administers the correct dose, adjusting for external factors such as heat, exercise and sleep.
The “artificial pancreas” hybrid closed loop (HCL) system represents the most significant breakthrough in T1D treatment since the discovery of insulin a century ago. The technology combines the use of an app, a continuous glucose monitor and an insulin pump to deploy an algorithm that learns each individual’s insulin needs and administers the correct dose, adjusting for external factors such as heat, exercise and sleep.
HCL has demonstrated its efficacy in reducing dangerous blood glucose highs and lows, contributing to the stabilisation of healthy levels. The reliability offered by the system dramatically reduces the number of decisions that people with T1D have to make each day, making management of their condition so much easier.
Since 2006, JDRF International has contributed £115m to funding 13 years’ worth of clinical trials as well as research among clinicians to identify and overcome any barriers to widespread adoption of the system. The charity’s approach is “device-agnostic and pro-choice”, meaning that it backed a number of different research projects in order to maximise the chances of success, and bring down the cost of the technology. As a result, there are now five HCL systems available in the UK.
But to be offered on the NHS, as with any new health technology, the National Institute for Health and Care Excellence (NICE) needed to approve its use. JDRF UK was critical in securing this approval, working with people living with T1D and other stakeholders, including healthcare professionals, researchers and industry partners, to present a comprehensive and well-rounded view of the impact and benefits of HCL. The charity also participated in NICE Health Technology Appraisal consultations – submitting responses, attending meetings, and engaging in discussions with the NICE committee to ensure that patient perspectives and research findings were appropriately considered.
HCL was approved in Scotland in 2022 and by NICE in England and Wales in 2023, and is now expected to become the first line of treatment for the majority of people living with T1D over the next five years. JDRF UK is still working closely with NHS England and medtech firms to speed up the rollout and uptake of the technology, and NICE’s approval has also led to other high-income countries being keen to secure HCL regulatory approval.
The Charity Awards judges were impressed by JDRF’s focus on working across all sectors to drive the project forward. Karin Woodley, CEO of Cambridge House, said it led a four-way partnership between the NHS, people with T1D, and the healthcare industry to facilitate a technological breakthrough that would be “profoundly more groundbreaking for the next generation or two”.
Charity Awards judge Dr Priya Singh, chair of NCVO, said JDRF not only contributed funding but worked tirelessly to “break down barriers and create the flight path to that innovation being able to be applied”.
She added: “They absolutely embodied the fact that research itself might be fabulous but makes absolutely no difference unless you can get it out to people.”
Kris Murali, chief financial officer at Royal NAAFI and a trustee at Diabetes UK, said this was a genuine medical breakthrough that would totally transform people’s lives. “It will make a fantastic difference to the way people manage their diabetes,” he said.
CC Reg no. 295716

